Abstract:
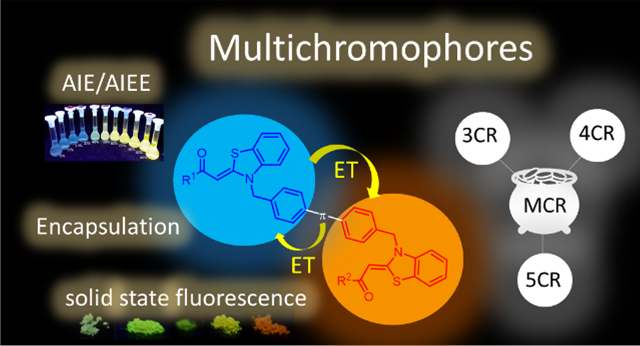
Asymmetrically bridged aroyl-S,N-ketene acetals and aroyl-S,N-ketene acetal multichromophores can be readily synthesized in consecutive three-, four-, or five-component syntheses in good to excellent yields by several successive Suzuki-couplings of aroyl-S,N-ketene acetals and bis(boronic)acid esters. Different aroyl-S,N-ketene acetals as well as linker molecules yield a library of
23 multichromophores with substitution and linker pattern-tunable emission properties. This allows control of different communication pathways between the chromophores and of aggregation-induced emission
(AIE) and energy transfer (ET) properties, providing elaborate aggregation-based fluorescence switches.
Zitat:
L. Biesen, J. Krenzer, N. Nirmalananthan-Budau, U. Resch-Genger,* T. J. J. Müller,* Asymmetrically bridged aroyl-S,N-ketene acetal-based multichromophores with aggregation-induced tunable emission. Chem. Sci. 2022, 13, 5374-5381. DOI: 10.1039/d2sc00415a