Hebah Fatafta, Mohammed Khaled, Michael C. Owen, Abdallah Sayyed-Ahmad, and Birgit Strodel
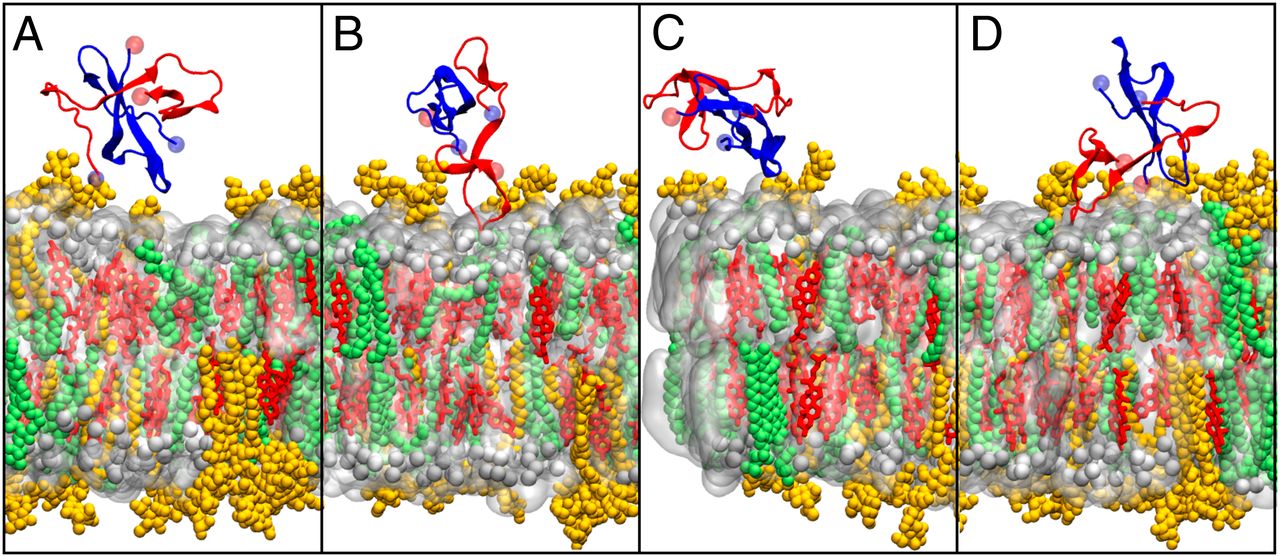
The aggregation of the amyloid-β peptide (Aβ) into neurotoxic oligomers is central to the development of Alzheimer’s disease. One possible source of their toxicity results from interactions of the Aβ oligomers with the neuronal membrane, damaging membrane integrity and thus neurons. However, molecular details of these interactions are unclear. Here, we contrast the dimerization of Aβ in solution and at the neuronal membrane. Our results clearly indicate that the sugar moieties of GM1 sequester Aβ by forming key hydrogen bonds with the peptide, which diverts the configuration of the Aβ dimers away from damaging β-sheet–rich structures. These findings underline the importance of GM1 in Alzheimer’s disease progression and provide a nanoscopic basis for its reported neuroprotective effect.